
Posts
Showing posts from November, 2023
- Get link
- X
- Other Apps
A liquid scintillation counter (LSC) is a device used to measure radioactivity in liquid samples. It works by detecting the scintillation light produced when a radioactive particle interacts with a liquid scintillator. The amount of scintillation light produced is proportional to the energy of the radioactive particle. There are two main types of LSC counters: Vial counters: These counters use individual vials to hold the sample and scintillator. Flow counters: These counters use a continuous flow of sample and scintillator.
- Get link
- X
- Other Apps

Amino acid synthesis is the set of biochemical processes ( metabolic pathways ) by which the amino acids are produced. The substrates for these processes are various compounds in the organism 's diet or growth media. Not all organisms are able to synthesize all amino acids. Primary amino acid synthesis: This is the synthesis of amino acids from simple precursors, such as alpha-ketoglutarate , pyruvate , and oxaloacetate. Primary amino acid synthesis is common to all organisms. Secondary amino acid synthesis: This is the synthesis of amino acids from other amino acids. Secondary amino acid synthesis is found in some, but not all, organisms.
- Get link
- X
- Other Apps
Autoradiography is a technique used to visualize the distribution of radioactive substances within a sample. DEFINITION Autoradiography is an imaging technique that uses radioactive sources contained within the exposed sample. It produces an image on an X-ray film or nuclear emulsion by the pattern of decay emissions (e.g., beta particles or gamma rays) from a distribution of a radioactive substance. Principle The principle of autoradiography is based on the interaction of radiation with photographic film. When a radioactive substance decays, it emits radiation that can strike silver halide crystals in the emulsion of a photographic film. Applications Visualizing the distribution of radiolabeled molecules in cells and tissues. Identifying radioactive contaminants in environmental samples. Types of autoradiography Contact autoradiography Emulsion autoradiography Advantages: Autoradiography is a sensitive technique that can detect very small amounts of radioactivity. Autoradi...
- Get link
- X
- Other Apps
Significance of Acetate Pathway The acetate pathway in plants is significant for several reasons Production of Phytoconstituents : The acetate pathway is important in the formation of various important phytoconstituents like fatty acids, polyketides, prostaglandins, aflatoxin, tetracycline, and other various important phytoconstituents. Flavonoid and Lipid Biosynthesis : The acetate pathway supports flavonoid and lipid biosynthesis in plants. Flavonoids are secondary metabolites involved in several physiological responses to the environment, such as defense against herbivores, UV radiation, and pathogens. Lipids are essential components of all cells, providing energy storage, insulation, and serving as structural components of cell membranes. Metabolic Sensor : Acetate acts, in part, as a metabolic sensor linking nutrient balance and cellular stress responses with gene transcription and the regulation of protein function. Fatty Acid Synthesis : The acetate pathway operates with the in...
- Get link
- X
- Other Apps
Secondary Metabolite: DEFINITION: Secondary metabolites are compounds that are not essential for the basic growth, development, and reproduction of organisms, but they may play important ecological roles. They are produced by a wide variety of organisms, including plants, fungi, bacteria, and insects. Secondary metabolites can have a wide range of biological activities, including: Defense, Communication between individuals, Attraction of pollinators, Allelopathy (the suppression of other plants) etc. Examples of secondary metabolites include: Alkaloids: morphine, caffeine, nicotine, quinine Terpenoids: essential oils, menthol, rubber Phenolics: flavonoids, tannins, lignin Quinones: ubiquinone, coenzyme Q10 Anthraquinones: laxatives, dyes Glycosides: cardiac glycosides, cyanogenic glycosides
- Get link
- X
- Other Apps
Potential Side effects of Antiplatelet Antiplatelet medications are generally well-tolerated, but they can cause a range of side effects. Here are some potential side effects associated with antiplatelet therapy: Common Side Effect : Bruising more easily Bleeding for longer Upset stomach Heavy periods in Females Nose bleeds Aspirin-Induced Asthma : This is a potential side effect of aspirin, the most commonly used antiplatelet drug. It can cause shortness of breath in some individuals. Hemorrhage : This is a severe side effect that involves excessive bleeding. It’s the main risk associated with antiplatelet therapy . Other Side Effects : These can include nausea , stomach pain , diarrhea , rash , and itching .
- Get link
- X
- Other Apps
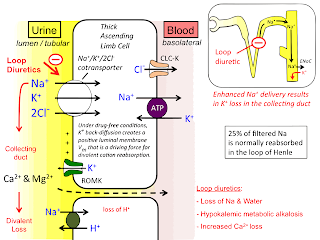
Diuretics: A diuretic is a substance that promotes diuresis, the increased production of urine . This can be a drug, or any other substance. Diuretics are often referred to as “ water pills ” and are used to increase the amount of water and salt excrete out from the body as urine. They are commonly used to treat conditions like high blood pressure and congestive heart failure. CLASSIFICATION in TERMS of Site of Action: Carbonic Anhydrase inhibitor: ( Post convoluted tubule )--- Acetazolamide Osmotic Diuretics: ( Descending limb of Henlie's Loop )--- Mannitol Loop Diuretics (Na+-K+-2Cl- Symporter inhibitors)/ High ceiling: ( Ascending Limb of Loop of Henle) --- Furosemide Inhibitors of Na+ Cl- symport (Thiazide and thiazide-like Diuretics): ( DCT ) --- Chlorothiazide K+ Sparing Diuretics (Inhibitors of renal epithelial Na+ channels): ( Collecting Tubule )--- Spironolactone How do loop diuretics, thiazide diuretics, and potassium-sparing diuretics...
- Get link
- X
- Other Apps
COLUMN CHROMATOGRAPHY Principle Column chromatography is a laboratory technique used to separate mixtures of compounds. It works by application of the absorbance of different rates at which the components of a mixture travel through a column packed with a stationary phase when driven by a solvent (the mobile phase). Procedure Prepare the column . Fill a glass column with a suitable stationary phase, such as silica gel or alumina. Pack the stationary phase tightly to prevent channeling, but not so tightly that it restricts the flow of solvent. Pre-elute the column. Add a small amount of solvent to the column and allow it to flow through. This will help to saturate the stationary phase and remove any air bubbles. Load the sample onto the column . The sample can be loaded onto the column in a variety of ways, but the most common method is to dissolve the sample in a small amount of solvent and add it to the top of the colum...
- Get link
- X
- Other Apps

MICROWAVE ASSISTED EXTRACTION Microwave-Assisted Extraction (MAE) is a sample preparation technique that uses microwave energy to rapidly heat and extract analytes from a sample matrix. MAE is a more efficient and effective extraction technique than traditional methods, such as Soxhlet extraction, because it uses less solvent and time. General Procedure The following is a general procedure for microwave-assisted extraction:Prepare the sample. Grind or homogenize the sample to a fine powder. Weigh the sample and solvent. A typical ratio of sample to solvent is 1:10. Place the sample and solvent in a microwave vessel . The vessel should be sealed to prevent solvent evaporation. Place the vessel in the microwave oven. Set the microwave power to 50-70% and the temperature to 100-120°C. Heat the sample for 5-10 minutes. The exact extraction time will depend on the sample matrix and analytes of interest. Allow the sample to cool. Filter the sample to remove the solid matrix. Coll...
- Get link
- X
- Other Apps

Isolation & identification of atropine & Quinine Isolation & identification of Atropine Isolation: Moisten the powdered bark with an aqueous solution of sodium carbonate and extract it with ether or benzene. The free alkaloids are extracted from the solvent and acidified with acetic acid. Discard the aqueous layer. Add sodium hydroxide to the organic layer to precipitate atropine sulfate. Filter, recrystallize, and purify the crystals with activated charcoal. Dissolve the atropine sulfate in dilute sulfuric acid and make it alkaline with ammonia. Atropine will precipitate as crystals. Wash and dry them at 45-55°C. Identification: Vitali-Morin Test: Atropine + Small Amount of Con. HNO3 Evaporated to dryness in Waterbath. Residue is dissolved in 1 ml Acetone and few drops of freshly prepared Alcoholic KOH, Observation: Violet Colour (Tropane Neuclus) Isolation & identification of Quinine Isolation Here are some steps for isolating ...
- Get link
- X
- Other Apps
ECUELLE METHOD The ecuelle method is a mechanical method of volatile oil extraction that is used primarily for citrus oils. Principle – In the ecuelle method, the citrus fruits are placed in a device with sharp projections on the inside. The device is then rotated, causing the projections to pierce the oil glands in the rind of the fruit. The essential oil is then released and flows down the sides of the device into a collection area. Procedure – Citrus fruit is washed and dried ¯ Placed in a device with sharp projections inside ¯ Device is rotated crushing the fruit oil glands ¯ Oil is released & collected Advantages – (i) More efficient than other methods (ii) Simple method (iii) Less cost...

%20(1).png)